Antioxidants: why the concept is wrongly understood
We hear everywhere that antioxidants are beneficial, but what is an antioxidant?
We hear everywhere that antioxidants are beneficial, but what is an antioxidant?
The word "antioxidant" is widely used in conversations about health and wellness, appearing in everything from dietary supplements to skincare items, with a worldwide market worth around $70 billion. The common belief is that antioxidants are always good for us, shielding our cells from harm and supporting a longer life.
However, this oversimplified view obscures the nuanced science behind antioxidants, oxidation, and their roles in human physiology. To fully grasp why antioxidants are both celebrated and misunderstood, we must first explore the fundamental chemistry and biology underlying these concepts.
What Is Oxidation?
At its essence, oxidation is a chemical reaction similar to combustion or burning. When a substance undergoes oxidation, it loses electrons to an oxidizing agent, often oxygen, resulting in energy release or molecular transformation. In everyday life, oxidation manifests as rust forming on iron or an apple browning after being cut. In the human body, oxidation is a critical process that sustains life. Our cells, particularly within the mitochondria, use oxygen to "burn" nutrients like glucose, producing adenosine triphosphate (ATP), the molecule that powers cellular functions. This controlled oxidative process is essential for energy production, much like a car engine combusting fuel to generate motion.
However, oxidation can also produce under certain conditions, harm to cells. This duality—oxidation as both vital and potentially damaging—sets the stage for understanding antioxidants.
Stages of oxidation: Rust - Fire - Explosion
Defining Antioxidants
An antioxidant is a substance that slows down or stops oxidation. Simply put, antioxidants stop or reduce the "burning" process by neutralizing reactive molecules that might otherwise damage cells. A familiar example is water: when it extinguishes a fire, water acts like an antioxidant by stopping the oxidative reaction. In living systems, antioxidants stabilize reactive molecules by donating electrons, preventing them from causing harm. Common antioxidants include vitamins such as C and E, minerals like selenium, and plant compounds found in fruits and vegetables.
But the question remains: Are antioxidants always beneficial, as popular narratives suggest?
In today's modern world, overweight and obesity represent some of the most serious and widespread health concerns, particularly in countries such as the United States of America, where sedentary lifestyles and high-calorie toxic diets have contributed significantly to this epidemic.
The conventional medical recommendation for individuals affected by excess body weight is often centered around the concept of "burning fat" through increased physical activity and exercise. This process, scientifically referred to as lipid peroxidation, involves the biochemical oxidation of fats (lipids) within the body .
But taking antioxidants during this process may actually hinder the necesary lipid peroxidation by neutralizing the free radicals required for it. However, this inhibition most likely decrease the effectiveness of burning fat . Therefore, the use of antioxidants might disrupt the body's natural fat-burning processes during exercise, underscoring the complexity of managing oxidative mechanisms in weight loss approaches.
The Free Radical Theory of Aging
In 1956, Denham Harman published a 1 and a half side paper introducing the free radical theory of aging, which posited that free radicals—molecules with unpaired electrons—are harmful to cells and contribute to aging and disease. This theory gained traction, because it was fueling the antioxidant industry’s growth.
Harman specifically highlighted hydroxyl radicals (OH), highly reactive molecules with an oxidation-reduction potential (ORP) of 2800 millivolts (mV) at pH 0 and 2300 mV at pH 7. ORP measures a substance’s ability to oxidize or reduce other molecules, with higher values indicating stronger oxidizing power. For comparison, oxygen, essential for cellular respiration, has an ORP ranging from 800 to 1280 mV—a range our cells are adapted to handle.
Context Dependence of ORP Values
It is important to note that oxidation-reduction potential (ORP) values are not fixed constants; they vary depending on environmental factors such as pH, temperature, and ionic strength. For example, the ORP of hydroxyl radicals is about 2800 mV at pH 0 but decreases to approximately 2300 mV at neutral pH 7. This contextual variability means that the oxidative or reductive power of a molecule must be understood relative to its biological or chemical environment, rather than as an absolute value.
Cells are like electronic devices, requiring a specific voltage to function optimally. Just as a smartphone needs 5 volts to charge without overheating, cells thrive within a particular ORP range. Hydroxyl radicals (OH*), with their excessively high ORP, can overwhelm cellular defenses, damaging proteins, lipids, and DNA if to much . This excessive oxidative stress is implicated in aging, cancer, and other diseases, as Harman’s theory suggested.
However, he general condemnation of free radicals is completely misleading. Not every free radical is damaging; their effects vary based on their oxidation-reduction potential (ORP) and the biological environment. Free radicals serve as crucial signaling molecules in functions like immune defense, cell growth, stem cell activation, and differentiation. The common oversimplification of free radicals as harmful has caused many misunderstandings about antioxidants.
Are Free Radicals Always Bad?
The harmfulness of a free radical hinges on its ORP rather than its status as a radical. A free radical is simply a molecule with an unpaired electron, making it highly reactive. This reactivity can be beneficial or detrimental, depending on the molecule’s voltage and the cellular environment. For instance, hydroxyl radicals are dangerous because their ORP (2300 mV at neutral pH) far exceeds the cellular norm, causing indiscriminate damage. In contrast, other radicals with lower ORPs may pose little threat or even serve physiological roles.
Diversity Among Free Radicals
The term “free radicals” broadly refers to molecules with unpaired electrons, but these radicals differ widely in their reactivity and biological roles. Some free radicals, such as nitric oxide (NO), serve essential signaling functions at physiological levels, while others like hydroxyl radicals are indiscriminately damaging due to their extreme reactivity. This diversity underscores why it is false to label all free radicals as harmful without considering their specific chemical nature and context within the cell.
Consider chlorine dioxide (ClO₂), a molecule often labeled a strong oxidizer and free radical. But with an ORP of 940 to 950 mV, ClO₂ it actually operates within the cellularly tolerable range. Its ability to accept four electrons makes it a strong oxidizer, but this strength is analogous to a car battery: It runs on a low voltage of 12V but provides a high current sufficient to start a truck, while still being safe to touch the contacts without risking injury. In contrast, an electric socket’s 220 V can cause severe injury due to its high voltage. Similarly, ClO₂’s reactivity allows it to target efectivly all kinds of pathogens due to their low ORP tolerances (as low as 90 mV) while sparing healthy cells, which are adapted to higher ORPs.
The Role of Antioxidants in Context
Antioxidants reduce oxidative stress by supplying electrons to reactive molecules, thereby decreasing their elevated ORP. This process, called reduction, is critical when hydroxyl radicals accumulate, such as during infections, excessive alcohol consumption, or chronic diseases like cancer. For example, after a night of heavy drinking, alcohol metabolism generates hydroxyl radicals, contributing to oxidative stress and the infamous hangover. This explains why the body craves antioxidant-rich foods like orange juice, which contains vitamin C and other reducing agents that mitigate this damage.
Defining Oxidative Stress
Oxidative stress occurs when there is an imbalance in the body favoring oxidants over antioxidants, leading to potential cellular damage. It is not simply the presence of free radicals or oxidizing agents but the failure of cellular antioxidant defenses to neutralize these reactive species adequately. Therefore, maintaining redox homeostasis—a delicate balance between oxidation and reduction—is crucial for healthy cell function and prevention of disease
However, antioxidants are not universally beneficial. Their efficacy depends on the specific oxidative challenge and the body’s physiological state. In cases of metabolic acidosis—when the body becomes overly acidic due to diet, stress, or disease—hydroxyl radical production increases, exacerbating cellular damage. Antioxidants can help by neutralizing these radicals, but excessive or inappropriate antioxidant use may disrupt normal cellular processes that rely on controlled oxidation.
Chlorine Dioxide: A Unique Case
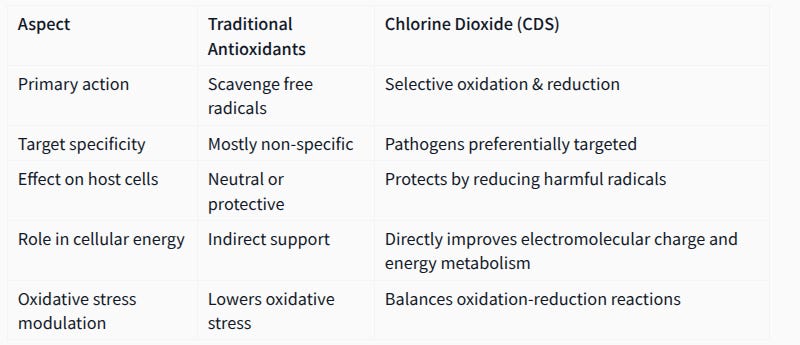
Chlorine dioxide (ClO₂), a significant health discovery, exemplifies the complexity of oxidative and reductive processes. Unlike traditional antioxidants, ClO₂ is an oxidizer with a dual role. Its ORP of 940–950 mV allows it to selectively oxidize pathogens with low ORP tolerances, such as bacteria or viruses, which typically have ORPs below 90 mV. Simultaneously, ClO₂ can reduce hydroxyl radicals by accepting their excess electrons, stabilizing their high ORP. Upon completing these reactions, ClO₂ degrades into harmless byproducts: table salt (NaCl) and oxygen (O₂). This dual action—oxidizing pathogens while reducing harmful radicals—makes ClO₂ a unique outstanding new therapeutic agent, though sadly its use remains controversial and requires financing for further research to be legalised.
The Misunderstood Role of Vitamin C
Vitamin C (ascorbic acid) is perhaps the most celebrated antioxidant, widely promoted for its ability to combat oxidative stress. However, high-dose vitamin C, such as 10-gram intravenous injections, reveals the complexity of antioxidants. At such doses, vitamin C acts as a pro-oxidant rather than an antioxidant, triggering the Fenton reaction. In this process, ascorbic acid reacts with iron or copper ions to produce hydrogen peroxide (H₂O₂), an oxidant with an ORP of 1760 mV.
While cells can tolerate this in the short term, H₂O₂ typically dissociates into hydroxyl radicals (OH*), which, with their high oxidation-reduction potential (ORP) of 2300 mV, then damage healthy cells. Thus, high-dose vitamin C may offer immediate benefits by targeting pathogens through oxidation but also poses risks of to much oxidative stress later, potentially causing harm.
This dual nature of vitamin C underscores a critical point: more is not always better. Many physicians administering high-dose vitamin C may not fully understand its pro-oxidant effects, leading to potential misuse. In contrast, dietary vitamin C requirements are modest. A daily intake of approximately 100 mg—equivalent to one apple or a small serving of citrus fruit—suffices to meet the body’s needs, supporting antioxidant defenses without risking oxidative imbalance.
Reframing Antioxidants
The antioxidant narrative, driven by Harman’s free radical theory and amplified by a $70 billion industry, has oversimplified a complex biological reality “ the more the better” that is false.
Antioxidants are neither universally good nor bad; their value depends on the context of oxidative stress, the specific radicals involved, and the body’s physiological state. Actually we do not know how it affect oxygen in our cells. Some scientists claim that Antioxidants reduce muscle effectiveness.
Free radicals like hydroxyl radicals can indeed harm cells when their ORP exceeds cellular tolerances, but not all radicals are detrimental, and some are essential for normal function. Substances like chlorine dioxide challenge the antioxidant paradigm by acting as both oxidizers and reducers, selectively targeting pathogens while mitigating harmful radicals.
Chlorine dioxide (ClO₂), particularly in its dissolved form known as CDS, challenges the traditional antioxidant paradigm by exhibiting a unique dual redox behavior:
it acts both as an oxidizer and a reducer !
Unlike conventional antioxidants that primarily neutralize free radicals by donating electrons, ClO₂ selectively targets pathogens through controlled oxidation of their electron-rich structures such as thiol groups and viral spike proteins (by oxidising their cystein and tyrosine), disrupting their vital functions without harming healthy cells.
Simultaneously, ClO₂ mitigates oxidative stress within host cells by reacting with and reducing harmful radicals like superoxide anions and hydroxyl radicals, converting them into Water( H₂O) and oxygen(O₂).
This very selective oxidation-reduction balance helps restore the electromolecular charge equilibrium essential for proper cellular energy metabolism and mitochondrial function. Thus, CDS supports recovery in various diseases by dynamically regulating the body’s redox environment—eliminating pathogens while protecting and revitalizing energy-depleted cells—offering a fundamentally new approach called electromolecular medicine. This mechanism underpins why chlorine dioxide is considered a significant medical discovery beyond traditional antioxidant therapies.
CDS can donate twice as many electrons as Vitamin C. Therefore, when taking CDS, there is no need for Vitamin C, and combining them does not make any sense.
Vitamin C highlights the need for a balanced and thoughtful approach.. While essential in small doses, excessive intake can shift it from an antioxidant to a pro-oxidant, with unintended consequences.
The key takeaway is balance: our cells require a delicate interplay of oxidation and reduction to thrive. By moving beyond the simplistic “antioxidants good, free radicals bad” narrative, we can better appreciate the intricate chemistry that sustains life and make informed choices about our health.
A deeper understanding of the body's electrical properties will shape the future. This is the foundation of electromolecular medicine.
If you want to know more about it join our online couses at Kalckerinstitute.com
All the best
Dr. h.c. Andreas Ludwig Kalcker
https://alkfoundation.com/en/
Ah….. my new Book is now availible in Voedia.com
Feel free to visit my oficial website: http://andreaskalcker.com for more information :))
Note: I do not sell any chemical substances or supplements. I am exclusively dedicated to electromolecular research. Unfortunately, some individuals have impersonated me on social media, selling products, cryptocurrency, or even paid consultations. Please be aware of fraud and use only the official websites linked at http://andreaskalcker.com







Interesting that all radicals are not bad...and antioxidants are not always beneficial.
Thank you so much for providing such a clear, comprehensive explanation of what is a nuanced, grossly misunderstood subject of health. I always felt suspicious of the ubiquity of antioxidant propaganda! Now I feel like I finally understand it! Bless you, Andreas. Please keep up the good fight of truth and health for humanity, educating the world on the veritable panacea that is Chlorine Dioxide! Truth prevails eventually, and so many of us have you to thank personally for our health and wellbeing 🙏🏼